Siegfried Eckert; Dieter Horstkotte. Published: 06/02/2009
Abstract and Introduction
Abstract
Progress in prevention as well as drug and interventional therapy has improved the prognosis of patients with cardiovascular disorders. Many patients at risk have advanced coronary artery disease (CAD), have had multiple coronary interventions, and present with significant co-morbidity. Despite adequate risk factor modulation and often several revascularization procedures, some of these patients still have refractory angina pectoris. Apart from advanced CAD and insufficient collateralization, the cause is often endothelial dysfunction. For this situation, one treatment option is neuromodulation. Controlled studies suggest that, in patients with chronic refractory angina pectoris, spinal cord stimulation (SCS) provides a relief from symptoms equivalent to that provided by surgical therapy, but with fewer complications and lower rehospitalization rates. SCS may result in significant long-term pain relief with improved quality of life. In patients with refractory angina undergoing SCS, some studies have shown not only a symptomatic improvement, but also a decrease in myocardial ischemia and an increase in coronary blood flow. Discussion is ongoing as to whether this is a direct effect on parasympathetic vascodilation or merely a secondary phenomenon resulting from increased physical activity following an improvement in clinical symptoms. Results from nuclear medical studies have sparked discussion about improved endothelial function and increased collateralization. SCS is a safe treatment option for patients with refractory angina pectoris, and its long-term effects are evident. It is a procedure without significant complications that is easy to tolerate. SCS does not interact with pacemakers, provided that strict bipolar right-ventricular sensing is used. Use in patients with implanted cardioverter defibrillators is under discussion. Individual testing is mandatory in order to assess optimal safety in each patient.
Introduction
Therapeutic options for the management of angina pectoris in patients with coronary artery disease (CAD) have improved over the past 2 decades. Nevertheless, angina pectoris is a common and important symptom affecting many patients with CAD, as well as some with endothelial dysfunction.
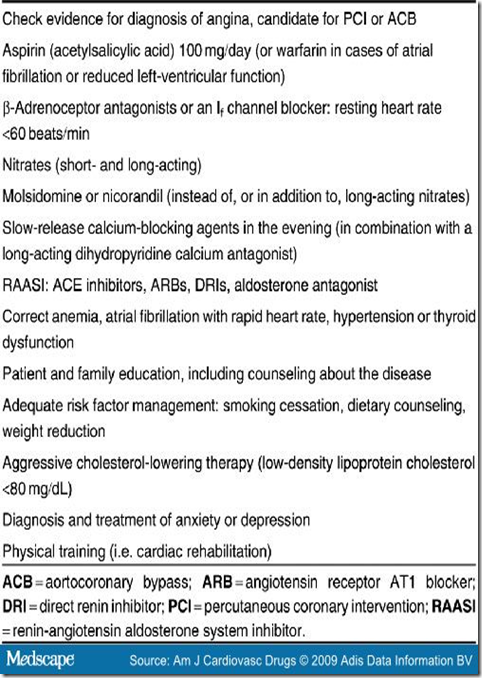
Despite optimal drug therapy and no option for coronary revascularization procedures (percutaneous coronary intervention [PCI] or aortocoronary bypass [ACB]), some patients with CAD have persistent angina pectoris class III or IV according to the Canadian Cardiovascular Society (CCS).[1] The treatment of these patients with non-responding angina pectoris presents a medical challenge. We have no accurate figures on the occurrence and frequency of refractory angina, nor is the prevalence of angina pectoris known in most communities. The overall prevalence of patients referred for coronary angiography with refractory angina varies from 5% to 15%.[2]
Various treatment concepts have been developed for patients with therapy-resistant angina pectoris and have been applied in clinical studies: long-term intermittent urokinase therapy,[3] surgical and percutaneous transmyocardial laser revascularization,[4-6] enhanced external counterpulsation,[7,8] percutaneous in situ coronary venous arterialization,[9] and transcutaneous electrical nerve and spinal cord stimulation (SCS).[10-19] The latter has been established as the most applicable. It is recommended as the therapy of choice by the European Society of Cardiology Joint Study Group on the Treatment of Refractory Angina.[2]
In this article, we review the role of SCS in the management of severe angina pectoris in patients with ischemic heart disease and endothelial dysfunction.
Endothelial Dysfunction and Coronary Artery Disease
Stable and Refractory Angina Pectoris
Endothelial dysfunction is usually diagnosed in the presence of angina pectoris without obstructive CAD and coronary artery spasm. Patients with such a diagnoses experience typical anginal chest pain and experience positive exercise stress testing. About 15-20% of patients undergoing cardiac catheterization for the assessment of typical chest pain have these characteristics,[20] and most of them have a good prognosis.[21]
Endothelial dysfunction often marks the onset of atherosclerosis, stays with the patient for the rest of his or her life, and at the end-stages of CAD following PCI, causes higher rates of relapse and re-intervention.[22] Endothelial dysfunction can be ascertained invasively and non-invasively. After excluding hemodynamically relevant epicardial stenoses by determining the fractional flow reserve (FFR), the functional status of a coronary artery can be determined by coronary flow reserve (CFR).[23] As a non-invasive procedure, ammonia positron emission tomography (PET)[24] and flow-mediated dilatation of the brachial artery (FMD) can be used.[22]
The survival of patients with CAD is increasing as a result of improved prevention and coronary intervention, which in turn is leading to an increase in the prevalence of patients with refractory angina pectoris.
It is important to underline that angina pectoris is a clinic diagnosis. Imbalance in myocardial oxygen demand and supply can produce myocardial ischemia. This may cause angina pectoris and lead to a reduction in left-ventricular contractility, as well as cause arrhythmia, myocardial infarction, and possibly death. Angina pectoris is commonly due to atherosclerosis of the coronary arteries, but it can also occur in conjunction with endothelial dysfunction due to insufficient coronary vasodilatation.
Anti-anginal drug therapy improves the imbalance of the myocardium by interacting with heart rate, cardiac pre- and afterload, as well as coronary vascular tone. Hemodynamically significant coronary stenoses with and without angina pectoris may be dilated (PCI) or operated on (ACB).[22]
Refractory angina pectoris (CCS class III and IV)[1] is a chronic condition characterized by the presence of angina due to coronary insufficiency in the co-presence of CAD that cannot be controlled by a combination of medical therapy, angioplasty, and coronary bypass surgery.[2] Patients with endothelial dysfunction can also experience refractory angina.
Before selecting patients with refractory angina for SCS, a re-evaluation of their medical therapy is required in order to ensure an optimal treatment regimen[2,25] (Table I). Myocardial ischemia should be present, and other causes of chest pain, such as musculoskeletal pain, esophageal reflux, gastrointestinal disorder, pericardial disease, vascular disease (aortic dissection, pulmonary embolism), infection, panic disorder, and pulmonary conditions that cause chest pain, must be excluded.[1,26]
Table I. Steps in Optimizing Medication and Management in Patients With Chronic Refractory Angina.
Pharmacologic Therapy
The therapeutic options for endothelial dysfunction and stable CAD are comparable and aim at correcting the imbalanced redox potential. Treating the classical risk factors with lifestyle modification and drug therapy has facilitated the successful prevention of clinical cardiovascular events and has prolonged life expectancy.
In many randomized studies, a reduction in increased low-density lipoprotein cholesterol and triglyceride, as well as an increase in reduced high-density lipoprotein cholesterol have contributed to stabilizing plaque, have frequently demonstrated a regression of coronary atherosclerosis, and have improved endothelial function.[22] Controlling hypertension, cessation of smoking, increasing physical activity, and reducing weight all help to halt the progress of atherosclerosis and to reduce acute events. Lifestyle changes in the form of increased physical activity with or without weight reduction frequently lead to a reduction in angina pectoris and improved myocardial perfusion.[22] In randomized studies, it was possible to show that, compared with coronary intervention (PCI), increasing physical activity resulted in a better event-free survival rate, a higher exercise capacity, and a higher oxygen uptake, and was more cost effective; it achieved clinical improvement in CCS class I at half the total cost of the interventional strategy.[27,28]
Spinal Cord Stimulation (SCS) in Angina Pectoris
Pathophysiologic Mechanisms of Pain
Pain arises when specific nerve endings in organs (nociceptors) are stimulated. Nociceptors end as free, non-corpuscular nerve endings in all the different types of tissue within our bodies, e.g. in the adventitia of coronary arteries. Angina pectoris results from ischemic episodes, reduced blood flow through hemodynamically significant stenoses of the epicardial vessels or endothelial dysfunction, stimulating chemosensitive and mechanoreceptive receptors in the heart. Activation of these receptors results in the release of prostaglandins, adenosine, bradykinin, and other substances that excite the sensory ends of the sympathetic and vagal afferent fibers.[29] The nociceptive information of the elicited stimulus that causes angina pectoris is conveyed by visceral afferent nerve fibers, converging in common pathways, into the dorsal spinal cord at C7-T5 level where they have synaptic connections with other neurons.[30] Afferent fibers from the heart and cutaneous input are assumed to converge on specific interneurons in the same segment of the spinal cord.[31] Angina pectoris is felt in areas that refer to the dermatome, from where afferent nerves project to the same segment of spinal cord as from the heart. Two different classes of fibers conduct the received signals to the central nervous system: thin medullated Aδ-fibers and non-medullated C-fibers. The majority of nociceptors are slow-conducting C-fibers that convey dull pain, whereas the fast-conducting Aδ-fibers convey stabbing pain.
SCS Techniques
During transcutaneous electric nerve stimulation (TENS), two or four adhesive electrodes are attached epicutaneously so that the induced paresthesia is located by the patient in the area with the highest projected pain intensity during typical angina pectoris. The stimulation intensity is selected below the individual pain threshold.
During SCS, a 4- or 8-pole electrode is advanced to C7/T1 using x-ray vision after puncturing of the epidural space under a local anesthetic at T6-7. The electrodes are placed according to the area of distribution of the electrically induced paresthesia. This area should correlate as closely as possible with the area affected by angina pectoris.[19,32] The stimulation electrode is guided under sterile conditions and connected to an external portable stimulation device followed by a positive test period, the duration of which depends on the frequency of angina. As soon as the frequency and intensity of angina pectoris have significantly decreased (more than 50%), the stimulation electrode is subcutaneously lengthened under general anesthesia and usually connected to a stimulation aggregate implanted in the left upper abdomen. Using a programming device, the patient can then switch the aggregate on and off telemetrically, as well as alter the stimulation intensity within stipulated ranges.
Mechanisms of Action of SCS in the Treatment of Cardiac Ischemic Syndromes
The following mechanisms may explain the positive effects of SCS in chronic refactory angina pectoris: reduced pain perception, decreased sympathetic tone, reduced myocardial oxygen demand, improved coronary microcirculatory blood flow, and positive effects on cerebral blood flow (CBF).[33]
Reduction of Pain Perception. Segmental pain inhibition through neurostimulation has been under discussion since the mid-1960s.[34] By applying electrical stimulation regionally and below the pain threshold, sensitive afferent fibers (A-fibers) are selectively activated. In the spinal dorsal horn, this should then lead to a consecutive presynaptic inhibition of nociceptive afferences (A- and C-fibers) with local analgesia. In the last few years, mechanisms involved in the modulation of important neurotransmitters have been discovered, contributing to our understanding of pain inhibition through SCS. SCS leads to an augmented release of the inhibitory neurotransmitter GABA. This results in a decrease in the release of exitatory amino acids (glutamate and aspartate). In a rat model, local infusion with a GABAb-receptor antagonist in the dorsal horn transiently abolished the SCS-induced suppression of glutamate and aspartate release. The elevation in GABA release, in response to SCS, did not reach statistical significance.[35,36] A combination of subtherapeutic intrathecal doses of a GABAb-receptor agonist and an adenosine A1 agonist has been found to potentiate the effects of SCS in rats that were not initially responsive to SCS.[35,36] SCS has been found to enhance the release of β-endorphin.[37] β-Endorphin can help to reduce pain perception. It can unfold a cardioprotective effect following myocardial ischemia by decreasing myocardial contractility and subsequent oxygen consumption, and possibly by decreasing the release of norepinephrine (noradrenaline).[38]
Decreased Sympathetic Tone. Several studies on the effect of TENS and SCS on cardiac metabolism and hemodynamics have demonstrated that no changes occur under basal conditions, but manifest when the heart is stressed.[39]
The anti-ischemic effect of SCS is not due to reduced cardiac sympathetic activity. SCS decreases overall sympathetic activity, which may benefit the heart, possibly by reducing oxygen demand.[11] This could be demonstrated with SCS during tachycardial atrial stimulation. The total body norepinephrine spillover was reduced while the cardiac norepinephrine spillover was not affected.[11] Regional and overall sympathetic activity can be differentiated using the isotope dilution technique.[40] In this technique, the norepinephrine spillover and clearance are determined simultaneously in a steady state by infusing tritium-labeled norepinephrine. This avoids an increase in cardiac norepinephrine spillover, which can happen when total body norepinephrine spillover is determined individually in addition to determination of the plasma concentration of endogenous norepinephrine.
Neurostimulation suppressed activity generated by intrinsic cardiac neurons in animal experiments.[41] Spinal cord neurons primarily influence the intrinsic nervous system via axons coursing the intrathoracic sympathetic nervous system. Transient regional myocardial ischemias can markedly increase the activity of the intrinsic nervous system and thus lead to cardiac dysrhythmia.[42] In sympathetic stress situations, SCS is able to lower heart rate.[43] This reduced cardiac sympathetic activity under SCS can also be ascertained in an influencing of heart rate variability.[44] Activation of spinal cord neurons during SCS induces a conformational change in the intrinsic cardiac nervous system that persists for a considerable period of time after termination. This remodeling of the intrinsic cardiac nervous system can override excitatory inputs to it arising from the ischemic myocardium.[45] Further studies are required to investigate whether ventricular tachycardia or sudden cardiac death could thus be avoided.
Effect on Cerebral Blood Flow. Non-invasive techniques for determining cerebral perfusion and measuring functional activity (e.g. PET, functional MRI, or magnetoencephalography) have contributed to a better understanding of cerebral function in healthy subjects and in those with various diseases and the ways in which cerebral function can be influenced through interventions.[45] Changes in regional blood flow in areas involved with nociception and cardiovascular control have been documented in patients treated with SCS for refractory angina.[46] The cerebral areas demonstrating a relative increase or decrease in CBF could possibly be determined by the underlying disease (pain during clinical examination, one-sided or two-sided for paired organs) and the measuring methods, as well as the type of intervention.[47,48] In patients with documented CAD, typical anginal and ischemic electrocardiographic changes can be triggered during dobutamine infusion. Dynamic PET examinations during induced ischemia reveal increased and decreased regional CBF.[48] During SCS, increased and decreased regional CBF can also be observed with and without stimulation in patients with refractory angina.[49] In these two different patient groups, there are correlations within the following regions: increased CBF in the hypothalamus, and in the periaqueductal grey area and bilaterally in the thalamus, and decreased CBF in the posterior insular cortex, an area that modulates sympathetic effects.[48,49] The well supported effects of SCS are possibly attained by influencing central structures within the area of pain perception and processing. The thalamus may act as a filter for afferent pain signals.[48,50]
Effect on Coronary Blood Flow. SCS has repeatedly demonstrated an anti-anginal effect by reducing angina pectoris and the use of short-acting nitrates, increasing exercise tolerance, and decreasing ST-segment depression on the electrocardiogram.[51-55] Discussion about the effect of SCS on myocardial blood flow is ongoing.
SCS has been demonstrated to reduce catecholamine levels.[56] A direct sympatholytic effect is under discussion. Stimulating the dorsal paths in the spinal cord leads in turn to stimulation of segmental reflex paths and an inhibition of tonic activity in the sympathetic nervous system.[57] Vasodilatation of the microvessels leads to improved myocardial perfusion. Even in patients with refractory angina pectoris, coronary reserve is not completely eliminated.[47] One study was able to demonstrate a significant increase in cardiac index and a decrease in pressure frequency product as an indirect indicator for a reduction in myocardial oxygen consumption.[46]
Changes in myocardial perfusion can be directly visualized by intracoronary pressure and flow measurements. Non-invasive methods, such as stress echocardiography and nuclear medical techniques like myocardial scintigraphy and PET, indirectly illustrate myocardial perfusion by measuring contractility of the left ventricle (wall motion analyses) and differences in activity of enriched nucleotides in the myocardial cells.
Not even the use of different methods to evaluate myocardial perfusion, with varying study designs and clear results, have been able to explain the key issue of the anti-ischemic effect of SCS.
In 15 patients, stress echocardiography with adenosine led to a slight decrease in pumping function during SCS, in comparison to baseline without stimulation.[58] This may be interpreted as an indirect indication of improved myocardial perfusion.
For direct measurement of blood flow in a coronary artery, a catheter or wire has to be introduced. This can contribute to a reduction in flow within the vessel, depending on the diameter of the catheter. The different studies employ a variety of systems and conditions. Chauhan et al.[57] examined 34 patients with syndrome X (endothelial dysfunction) and 15 patients with CAD. Measurements were taken using an 8-French catheter in a presumed healthy vessel - left coronary artery without significant stenoses - at rest, with and without neurostimulation (TENS). An increase in flow velocity was ascertained. The authors concluded that the site of action was at the microcirculatory level, and that the effects may be mediated by neural mechanisms.
Norrsell et al.[59] examined eight patients with advanced CAD and four patients with syndrome X. A Doppler guidewire was placed in the vessel, corresponding to the ischemic area on a prior myocardial scintigram. Perfusion at rest and with right-ventricular stimulation, with and without SCS, was examined. The result was negative. There were no significant changes in coronary flow velocity during maximum pacing frequency when stimulation was introduced.
In a prospective study in 31 patients with advanced CAD, Diedrichs et al.[60] demonstrated an improvement in myocardial perfusion with sestamibi-single-photon emission computed tomography (MIBI-SPECT) scintigraphy after 12 months, but not after 3 months. Thus the reduction in ischemia does not seem to be a direct effect of neurostimulation, but might be due to an increased exercise tolerance of the patients with improved cardiac blood flow because of a better collateralization.
PET examinations of myocardial perfusion with SCS have also revealed different results. In eight patients undergoing SCS, De Landsheere et al.[61] found no increase in regional myocardial perfusion in ischemic regions when stimulated. Hautvast et al.[62] postulated a homogenization of myocardial blood flow. In nine patients with CAD, no significant difference in coronary blood flow due to SCS was revealed during dipyridamole stress testing after 6 weeks of SCS. Total resting blood flow remained unchanged, but flow reserve decreased. In a pilot study, we examined six patients with advanced CAD.[63] Perfusion at rest and during the maximum hemodynamic effect of intravenous adenosine was studied by ammonia PET at baseline and 13 ± 0.5 months after SCS. We found a significant increase in myocardial blood flow and a reduction in minimal coronary resistance after 1 year.[63] Figure 1 shows increased myocardial perfusion in the basal posterior wall of a 62-year-old man undergoing SCS. His history is typical for patients with refractory angina pectoris: severely restricted left-ventricular function in conjunction with advanced coronary triple vessel disease, two myocardial infarctions, two operative myocardial revascularizations, three catheter interventions, all therapeutic options exhausted, and angina upon slight physical exercise. In all patients, additional 18 F-fluorodeoxyglucose positron emission tomography ( 18 F-FDG-PET) was performed at baseline to distinguish vital myocardial regions from non-vital regions (figure 2). Fifty patients are included in an ongoing prospective study of the same design.[64]
Figure 1. Improvement in myocardial blood flow in the inferior wall of the heart in a 65-year-old man after 1 year of spinal cord stimulation. (a) Baseline examination; and (b) 1-year follow-up.
Figure 2. Assessment of viability by positron emission tomography (PET) imaging. Baseline examination: (a) discrimination between vital and non-vital myocardium using 18 F-fluorodeoxyglucose positron emission ( 18 F-FDG-PET). Detection of ischemia by ammonia PET at (b) rest and (c) stress.
The results pertaining to improved myocardial perfusion following SCS are not uniform. The number of patients included in studies is small, and follow-up intervals differ. A direct effect of neuromodulation on myocardial perfusion is not conclusive, even with a positive result. The discussion addresses a relative redistribution of myocardial perfusion from non-ischemic to ischemic myocardial areas when stimulated. Since the differences are slight and the reduction in myocardial perfusion in the non-ischemic areas presumes a significantly higher level than the increase to be achieved in the ischemic areas, ischemia is not to be expected in the non-ischemic areas. In the ischemic areas, however, a significant increase in perfusion is to be expected. This explains the symptomatic improvement, which may be explained by a direct effect due to vasodilatation, in particular, of the microvessels, with a reduction in minimum coronary resistance, and an indirect effect due to an improved collateralization (development of collateral vessels).
Improvement in myocardial perfusion through an indirect improvement in endothelial function can be explained by the presumed increase in exercise tolerance and quality of life when angina pectoris is reduced.[27] Whether or not the anti-anginal and anti-ischemic effects of SCS are mediated by an increase in coronary flow velocity is a discussion still ongoing. The effect is derived through decreased myocardial oxygen consumption.[11]
Clinical Experience with SCS in Angina Pectoris
Angina pectoris is a projected pain that is caused by insufficient perfusion of the myocardium due to significant coronary stenoses or reduced vasodilatatory capacity, particularly of the microcirculation. CAD is frequently accompanied by endothelial dysfunction. In the majority of patients with significant coronary stenoses and exercise-induced ischemia, pain relief can be achieved following revascularization and/or through risk factor modulation with improvement in endothelial function (Table I). TENS and SCS are recognized therapies in patients with refractory angina pectoris.[2] In many clinical studies, the effectiveness of these therapies have been demonstrated in patients (approximately 2500) with CAD and endothelial dysfunction:[2,21,54,55,59,65-70] fewer angina pectoris episodes and less short-acting nitroglycerin (glyceryl trinitrate) or mononitrate intake per time period; increase in exercise tolerance, time to angina, and the appearance of ST-segment depression; extended walking distance in the 6-minute walk test before onset of angina; improvement in quality of life; and fewer stays in hospital as well as visits to the physician due to cardiac-related symptoms.
Table I. Steps in Optimizing Medication and Management in Patients With Chronic Refractory Angina.
Despite these well known symptomatic improvements, SCS is still not recognized by many cardiologists. Its acceptance in European countries is low and varies (countries employing SCS in descending order of frequency are: Sweden, Italy, Germany, and Denmark). Since to date there are no systematic investigations regarding the distribution of SCS in patients with refractory angina pectoris, statistics come from manufacturers only (total 400-500 patients/year; in Germany, a total of 225 patients since 1998).
In a randomized, prospective study, the effectiveness of SCS was compared with that of operative myocardial revascularization (ACB).[18] SCS (n = 53, 41 males) and ACB (n = 51, 42 males) scored equally in subjective symptomatic improvement. Compared with the SCS group, after 6 months of follow-up, the ACB group had an increased exercise tolerance, as well as less ST-segment depression on maximum and comparable workloads. The maximum workload capacity was lower in the SCS group, and the ST-segment depression on maximum workload was higher than that in the ACB group. The mortality rate was lower in the SCS group (one SCS patient vs seven ACB patients). After 5 years, survival and quality of life were comparable between the two groups.[19] Secondary prevention was poor in both groups (SCS/ACB): aspirin (acetylsalicylic acid) 42%/33%, β-adrenoceptor antagonists 43%/24%, lipid-lowering drugs 6%/3% and ACE inhibitors 7%/8%.
Since patients experience retrosternal prickling during active SCS, a blind, placebo-controlled SCS study in patients with refractory angina pectoris is not an option. Eddicks and coworkers[54] have examined the therapeutic effects of subthreshold SCS. Twelve responders to SCS were randomized into four consecutive treatment arms, each for 4 weeks, with various stimulation timing and output parameters. One group was defined as a control-subthreshold stimulation (0.1 V) without retrosternal prickling. Walking distance, angina pectoris, short-acting nitroglyerin intake, and quality of life only improved in the groups sensing stimulation. This study showed for the first time that a placebo effect in conjunction with SCS is unlikely. The option of using subthreshold stimulation in active yet blind patient treatment is an attractive concept for further studies.
Safety Aspects
SCS is a safe, recognized, and effective therapy for patients with refractory angina pectoris.[2,70-73] Critics of SCS object that patients no longer receive warning signals (angina pectoris) and thus endanger themselves as their exercise levels increase.[74] Angina pectoris is the cardinal symptom of acute myocardial ischemia including infarction, and it is possible that effective pain relief through SCS may conceal such an infarction.[75] In many studies, it has been shown, however, that angina pectoris could be significantly reduced with SCS. Quality of life improves and cardiovascular event rates are significantly lower.[12,70,76,77] Of course, myocardial infarctions cannot be prevented through SCS: advancing CAD, plaque ruptures, or bypass closures can still be responsible for these.
In a prospective study, Andersen and coworkers[74] investigated the possibility that SCS used for pain relief might conceal acute myocardial infarction. During the observation period of up to 37 months (108.6 patient-years), ten out of 50 patients experienced a myocardial infarction. Nine of these ten patients with acute infarction recognized that the precordial pain was clearly different and definitely more severe than their usual angina. Angina was not influenced by SCS. The mean number of admissions for chest pain, angina, or observation, in case of acute myocardial infarction, was not significantly different in the ten patients with acute myocardial infarction compared with patients without infarction during the 3-year period before SCS treatment or during SCS treatment.
SCS is used in patients with advanced CAD. Patients with CAD can also experience disorders that necessitate the use of permanent pacemaker (PPM) treatment for bradyarrhythmias or an implantable cardioverter defibrillator (ICD) for ventricular tachycardias.
The experiences of various groups confirm the safety of SCS in patients with PPM.[78-84] SCS does not interact with pacemakers, provided that strict bipolar right-ventricular sensing is used. Unipolar SCS has been reported to cause PPM inhibition, and should not be considered.[7,81] The amplitudes of the stimulator noise are often seen on the intracardiac electrogram (figure 3). There were no interactions between the two systems during T-wave sensing with unipolar PPM. Individual testing is mandatory to assess safety in each patient.
Figure 3. Intracardiac electrograms showing stimulator noise amplitudes in patients with permanent pacemakers undergoing spinal cord stimulation (SCS): no noise interference.
In the future, more patients with refractory angina will have pacemakers (including left-ventricular stimulation) and ICDs. An increase in patients with ICDs has already been observed over the past decade. In line with current information, many patients included in ESBY (Electrical Stimulation versus Bypass Surgery in Severe Angina Pectoris) study have an indication for ICD therapy due to severely impaired left-ventricular function. Thus, the treatment of one condition must be compatible with the treatment of the others.
The literature only contains case studies of combined SCS and ICD therapy.[85] Problems can arise, on the one hand, from a false detection of SCS spikes with consecutive antitachycardia therapy and, on the other hand, from the suppression of therapy in conjunction with a threat of arrhythmia, since spikes are often still falsely interpreted as 'rhythm' below the intervention threshold.
Many ICD systems use an automated gain-control during bipolar sensing. According to our experience, ICD-SCS combination therapy may be safely performed.[86,87] Differentiated testing is unavoidable. In a prospective study in five patients within a follow-up of 12.2 ± 10.5 (2-40) months, no interaction between SCS and ICD therapy was documented.[87] We evaluated possible interactions under general anesthesia with the highest SCS amplitude (10.5 V/450 ms) and the most sensitive bipolar sensing during induced ventricular fibrillation (twice) [figure 4].
Figure 4. Spinal cord stimulation (SCS) and implantable cardioverter defibrillator therapy: no interaction by induction (a) and termination (b) of ventricular fibrillation (highest sensitivity).
TENS therapy should not be performed in patients in conjunction with SCS, PPM, cardiac resynchronization therapy (CRT), or ICD since inhibitions cannot be excluded. The general recommendations regarding minimization of interactions and infections should be observed for SCS as for other stimulation therapies. MRI and diathermia should not be performed due to possible warming of the leads.
Stress tolerance in patients with SCS is not limited by the wearing of the system, but by progressive CAD, restricted left-ventricular function, and possible concomitant diseases.
Cameron[55] has summarized direct SCS-related complications from the literature over the last 20 years, covering a total of 2753 patients: lead migration 13.2%, lead breakage 9.1%, and infection 3.4%. Through optimization of implantation technique and perioperative management, these complications can be reduced.[80,81] In the German Angina Register[76,88] including 101 patients, the frequency of these complications is as follows: lead migration 5%, lead breakage 5%, and infection 3%. In the 1-year follow-up, 8% died: sudden cardiac death occurred in 3%, heart failure in 2%, and malignoma in 3%. One patient experienced a myocardial infarction and one patient underwent PCI.
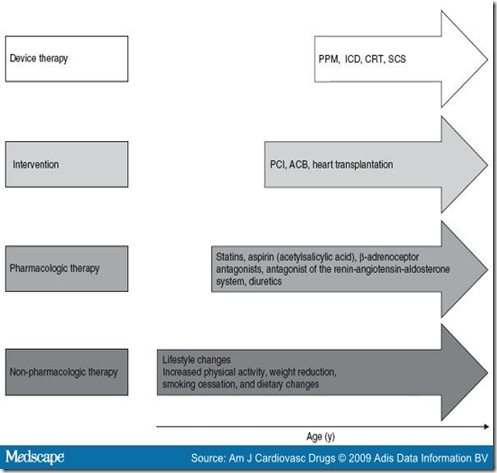
SCS shows a long-term beneficial effect, even in patients with unstable angina.[51] The target values of concomitant risk factors must be reduced prior to commencing SCS and then be aligned long-term through interventions (Table I, figure 5).
Table I. Steps in Optimizing Medication and Management in Patients With Chronic Refractory Angina.
Figure 5. Therapeutic options in the cardiovascular continuum for patients with unstable angina. ACB = aortocoronary bypass; CRT = cardiac resynchronization therapy; ICD = implantable cardioverter defibrillator; PCI = percutaneous coronary intervention; PPM = permanent pacemaker; SCS = spinal cord stimulation.
Conclusion
Refractory angina pectoris during end-stage CAD and with endothelial dysfunction is a specific coronary syndrome that is chiefly caused by microcirculatory disturbances. Already receiving the best use of evidence-based therapies and with no interventional options, these patients can benefit from SCS. SCS is safe and effective for treating refractory angina pectoris - reducing both the number of anginal episodes and the intensity of the angina pectoris. With SCS the dosage of short-term effective nitrates per time period is reduced. The work period during exercise tests is significantly prolonged. SCS leads to a significant reduction in hospital admission for cardiac causes, without masking myocardial ischemias or myocardial infarction. The implantation costs are balanced out by savings in aftercare (fewer consultations and hospital stays).
SCS is an excellent alternative for patients at an increased risk of requiring operative revascularization. For patients with refractory angina who are waiting for heart transplantation, SCS is also a good bridging option.
In small studies, an improvement in myocardial blood flow in vital ischemic myocardial areas has also been proved. It has yet to be investigated whether SCS, in addition to a proven improvement in symptoms, also reduces mortality.
References
- Campeau L. Grading of angina pectoris. Circulation 1976; 54: 522-3
- Mannheimer C, Camici P, Chester MR. The problem of chronic refractory angina. Eur Heart J 2002; 23: 355-70
- Leschke M, Schoebel FC, Mecklenbeck W. Long-term intermittent urokinase therapy in patients with end-stage coronary artery disease and refractory angina pectoris: a randomized dose-response trial. J Am Coll Cardiol 1996; 27: 575-84
- Burkhoff D, Schmidt S, Schulman SP. Transmyocardial laser revascularisation compared with continued medical therapy for treatment of refractory angina pectoris: a prospective randomised trial. Lancet 1999; 354: 885-90
- Bridges CR, Horvath KA, Nugent WC. The Society of Thoracic Surgeons practice guideline series: transmyocardial laser revascularization. Ann Thorac Surg 2004; 77: 1494-502
- Oesterle SN, Sanborn TA, Resar J. Percutaneous transmyocardial laser revascularisation for severe angina: the PACIFIC randomised trial. Lancet 2000; 356: 1705-10
- Stys TP, Lawson EW, Hui JCK. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol 2002; 89: 822-4
- Sinvhal RM, Gowda RM, Khan IA. Enhanced external counterpulsation for refractory angina pectoris. Heart 2003; 89: 830-3
- Oesterle SN, Reifart N, Haupftmann E. Percutaneous in situ coronary venous arterialisation report of the first human catheter-based coronary artery bypass. Circulation 2001; 103: 2539-43
- Murphy DF, Giles K. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain 1987; 28: 365-8
- Mannheimer C, Eliasson T, Andersson B. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ 1993; 307: 477-80
- Eliasson T, Jern S, Augustinsson LE, et al. Safety aspects of spinal cord stimulation in servere angina pectoris. Coron Artery Dis 1994; 5: 845-50
- de Jongste MJL, Nagelkerke D, Hooyschnuur CM, et al. Stimulation characteristics, complications, and efficacy of spinal cord stimulation systems in patients with refractory angina. Pacing Clin Electrophysiol 1994 Nov; 17 (11 Pt 1): 1751-60
- Oosterga M, Vaarwerk ten IAM, de Jongste MJL. Spinal cord stimulation in refractory angina: clinical results and mechanisms. Z Kardiol 1997; 86 (1 Suppl.): 107S-13S
- Hautvast RWM, de Jongste MJL, Staal MJS. Spinal cord stimulation in chronic intractable angina pectoris: a randomised, controlled efficacy study. Am Heart J 1998; 136: 1114-20
- Greco S, Auriti A, Fiume D. Spinal cord stimulation for the treatment of refractory angina pectoris: a two-year follow-up. Pacing Clin Electrophysiol 1999; 22 (Pt 1): 26-32
- Di Pede F, Lanza GA, Zuin G. Immediate and long-term clinical outcome after spinal cord stimulation for refractory stable angina pectoris. Am J Cardiol 2003; 91: 951-5
- Mannheimer C, Eliasson T, Augustinsson LE. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris. The ESBY study. Circulation 1998; 97: 1157-63
- Erke O, Eliasson T, Norrsell H. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J 2002; 23: 1938-45
- Kemp HG, Kronmal RA, Vliestra RE. Seven year survival of patients with normal or near normal coronary angiograms: a CASS registry study. J Am Coll Cardiol 1986; 7: 476-83
- Opherek D, Schuler G, Watterauer K. Four-year follow up study in patients with angina pectoris and normal coronary angiograms (syndrome X). Circulation 1989; 80: 1610-6
- Patti G, Pasceri V, Melfi R. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation 2005; 111: 70-5
- Pijls NHJ, De Bruyne B, Smith L. Coronary thermodilution to assess flow reserve. Circulation 2002; 105: 2482-6
- Pijls NHJ, Klauss V, Siebert U. Coronary pressures measurement after stenting predicts adverse events at follow-up. Circulation 2002; 105: 2950-4
- Fox K, Alonso Garcia MA, Ardissino D, et al., for the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. ESC guidelines for the management of stable angina pectoris: executive summary. Eur Heart J 2006; 27: 1341-81
- Lee TH, Cannon CP. Approach to the patient with chest pain. In: Zipes DP, Libby P, Bonov RO, et al., editors. Braunwald's heart disease. Philadelphia (PA): Elsevier Saunders, 2005: 1129-39
- Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease. Circulation 2004; 109: 1371-78
- Belardinelli R, Georgiou D, Ginzton L. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 1998; 97: 553-61
- Bolser DC, Chandler MJ, Garrison DW. Effects of intracardiac bradykinin and sapsaicin on spinal and spinoreticular neurons. Am J Physiol 1989; 257: 1543-50
- Foreman RD, Ohata CA. Effects of coronary artery occlusion on thoracic spinal neurons receiving viscerosomatic inputs. Am J Physiol 1980; 238: 667-74
- Selzer M, Spencer WA. Interactions between visceral and cutaneous afferents in the spinal cord: reciprocal primary afferent fiber depolarization. Brain Res 1969; 14: 349-66
- Sanderson JE, Brooksby P, Waterhouse D. Epidural spinal electrical stimulation for severe angina pectoris. Eur Heart J 1992; 13: 628-33
- Latif OA, Nedeljkovic SS, Stevenson LW. Spinal cord stimulation for chronic intractable angina pectoris: a unified theory on its mechanism. Clin Cardiol 2001; 24: 533-41
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150: 971-9
- Cui JG, O'Connor WT, Ungerstedt U, et al. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1997; 87: 87-95
- Cui JG, Meyerson BA, Sollevi A, et al. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABAB and adenosine receptor activation. Neurosci Lett 1989; 247: 183-7
- Oldroyd KG, Harvey K, Gray CE, et al. Beta-endorphin release in patients after spontaneous and provoked acute myocardial ischaemia. Br Heart J 1992; 67: 230-5
- Eliasson T, Mannheimer C, Waagstein F, et al. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology 1998; 89: 170-7
- Mannheimer C, Carlsson CA, Emanuelsson H, et al. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation 1985; 527: 11-6
- Norsell H, Elissson T, Mannheimer C, et al. Effects of pacing-induced myocardial stress and spinal cord stimulation on whole body and cardiac norepinephrine spillover. Eur Heart J 1997; 18: 1890-6
- George MS, Sackeim HA, Rush AJ, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatr 2000; 47: 287-95
- Elser M, Jennings G, Korner B, et al. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol 1984; 247: E21-8
- Foreman RD, Linderoth B, Ardell JF, et al. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 2000; 47: 367-75
- Olgin JE, Takahashi T, Wilson E, et al. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and artrioventricular nodes. J Cardiovasc Electrophysiol 2002; 13: 475-81
- Moore R, Groves D, Nolan J, et al. Altered short term heart rate variability with spinal cord stimulation in chronic refractory angina: evidence for the presence of procedure related cardiac sympathetic blockade. Heart 2004; 90: 211-2
- Emanuelsson HC, Mannheimer F, Waagstein C, et al. Catecholamine metabolism during pacing-induced angina pectoris and the effect of transcutaneous electrical nerve stimulation. Am Heart J 1987; 114: 1360-6
- Strauer BE. Ventricular function and coronary hemodynamics in hypertensive heart disease. Am J Cardiol 1979; 44: 999-1018
- Haustvast RWM, Ter Horst GJ, DeJong BM, et al. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. European Journal of Neuroscience 1997; 9: 1178-83
- Zonenshayn M, Mogilner AY, Rezai AR. Neurostimulation and functional brain imaging. Neurol Res 2000; 22: 318-25
- Rosen SD, Paulesu E, Firth CD, et al. Central nervous pathways mediating angina pectoris. Lancet 1994; 344: 147-50
- DeVries J, DeJongste MJL, Zijlstra F, et al. Long-term effects of electrical neurostimulation in patients with unstable angina. Refractory to conventional therapies. Neuromodulation 2007; 10: 345-8
- Andersen C, Enggard TP, Scherer C, et al. Spinal cord stimulation has a proven benefit on pain and quality of life in patients with angina pectoris when less invasive therapies have failed. Neuromodulation 2006; 9: 314-9
- Sanderson JE, Ibrahim B, Waterhouse D, et al. Spinal electrical stimulation for intractable angina - long-term clinical outcome and safety. Eur Heart J 1994; 15: 810-4
- Eddicks S, Maier-Hauff K, Schenk M, et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart 2007; 93: 585-90
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20 year literature review. J Neurosurg 2004; 100: 254-67
- Schoebel FC, Leschke M, Strauer BE. Therapierefraktäre angina pectoris. Dtsch Med Wschr 1995; 120: 301-7
- Chauhan A, Mullins PA, Thuraisingham G, et al. Effects of transcutaneous electrical nerve stimulation in coronary blood flow. Circulation 1994; 89: 694-702
- Kujacic V, Eliasson T, Mannheimer C, et al. Assessment of the influence of spinal cord stimulation on left ventricular function in patients with server angina pectoris: an echocardiographic study. Eur Heart J 1993; 14: 1238-44
- Norrsell H, Eliasson T, Albertsson P, et al. Effects of spinal cord stimulation on coronary blood flow velocity. Coron Artery Dis 1998; 9: 273-8
- Diedrichs H, Weber M, Koulousakis A, et al. Improved myocardial blood flow in patients with intractable angina pectoris through spinal cord stimulation. J Nucl Cardiol 2004; 11 Suppl. 4: 25S
- De Landsheere C, Mannheimer C, Habets A, et al. Effect of spinal cord stimulation on regional myocardial perfusion assessed by positron emission tomography. Am J Cardio 1992; 69: 1143-9
- Hautvast RW, Blanksma PK, DeJongste MJL, et al. Effects of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol 1996; 77: 462-7
- Wielepp JPP, Eckert S, Dongas A, et al. Effect of spinal cord stimulation on myocardial blood flow in patients with refractory angina pectoris. J Nucl Cardiol 2005; 12 (Suppl.): 64S
- Fricke E, Eckert S, Dongas A, et al. Myocardial perfusion after one year of spinal cord stimulation in patients with refractory angina. Nuklearmedizin. In press
- Eliasson T, Albertsson P, Hardhammar P, et al. Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron Artery Dis 1993; 4: 819-27
- Kaski JC, Russo G. Microvascular angina patients with syndrom X. Z Kardiol 2000; 89 Suppl. 9: 121-5
- Lanza GA, Sestito A, Sgueglia GA, et al. Effect of spinal cord stimulation on spontaneous and stress-induced angina and 'ischemia-like' ST-segment depression in patients with cardiac syndrome X. Eur Heart J 2005; 26: 983-9
- Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart 2007; 93: 159-66
- Jessurun GAJ, Hautvast RWH, Tio RA, et al. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: fad or future? Eur J of Pain 2003; 7: 507-12
- DeVries J, DeJongste MJL, Versteegen GJ, et al. Personality: predictor of neurostimulation outcomes in patients with chest pain and normal coronary artery. Neuromodulation 2006; 9: 123-5
- DeJongste MJL, Haaksma J, Hautvast RWM, et al. Effects of spinal cord stimulation on myocardial ischaemia during daily life in patients with severe coronary artery disease. Br Heart J 1994; 71: 413-8
- Gruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neuro 2007; 10: 1331-50
- Loeser JD. Evidence-based medicine and neuromodulation. Neuromodulation 2008; 11: 151-5
- Andersen C, Hole P, Oxhoj H. Does pain relief with spinal cord stimulation conceal myocardial infarction? Br Heart J 1994; 71: 419-21
- Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006; 58: 481-96
- Eckert S, Theres H, Eddicks S. Wie sicher ist die spinal cord stimulation zur Behandlung der therapierefraktären angina pectoris. Clin Res Cardiol 2007; 96 Suppl. 1: 523
- TenVaarwerk IA, Jessurun GA, DeJongste MJL, et al. Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris. Heart 1999; 82: 82-8
- Erke O, Börjesson M, Edvardsson N, et al. Feasibility of spinal cord stimulation in angina pectoris with chronic pacemaker treatment for cardiac arrhythmias. Pace 2003; 26: 2134-41
- Andersen C, Oxhoj H, Arnsbo P. Management of spinal cord stimulation in patients with cardiac pacemakers. Pace 1990; 13: 574-7
- Romano M, Zucco F, Baldini MR, et al. Technical and clinical problems in patients with simultaneous implantation of a cardiac pacemaker and a spinal cord stimulator. Pace 1993; 16: 1639-44
- Iyer R, Gnanadurai TV, Forsey P. Mangement of cardiac pacemaker in a patient with spinal cord stimulator implant. Pain 1998; 74: 333-5
- Kumar K, Buchser E, Linderoth B, et al. Avoiding complication from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation 2007; 10: 24-33
- Pinski SL, Trohman RG. Interference in implanted cardiac devices. Pace 2002; 25: 1367-81
- Kosharskyy B, Rozen D. Feasibility of spinal cord stimulation in a patient with cardiac pacemaker. Pain Physician 2006; 9: 249-52
- Schimpf R, Wolpert C, Herwig S, et al. Potential device interaction of a dual chamber implantable cardioverter defibrillator in a patient with continuous spinal cord stimulation. Europace 2003; 5: 397-402
- Ferrero P, Grimaldi R, Massa R, et al. Spinal cord stimulation for refractory angina in a patient implanted with a cardioverter defibrillator. Pacing Clin Electrophysiol 2007; 30: 143-6
- Eckert S, Dongas A, Güldner H, et al. Immediate and long-term clinical outcome after spinal cord stimulation for refractory stable angina pectoris in patients with chronic pacemaker- and ICD-treatment. Eur Heart J 2006; 27 Suppl. 6: 463S
- Theres H, Eckert S, Eddicks S. Spinal cord stimulation (SCS) zur Behandlung der refraktären angina pectoris in Deutschland - Ergebnisse nach 12 Monaten Verlauf im Rahmen des Deutschen SCS Registers. Clin Res Cardiol 2007; 96 Suppl. 1: 522
Authors
Siegfried Eckert and Dieter Horstkotte, Department of Cardiology, Heart and Diabetes Center North Rhine-Westphalia, Ruhr-University Bochum, Bad Oeynhausen, Germany











0 comments:
Post a Comment